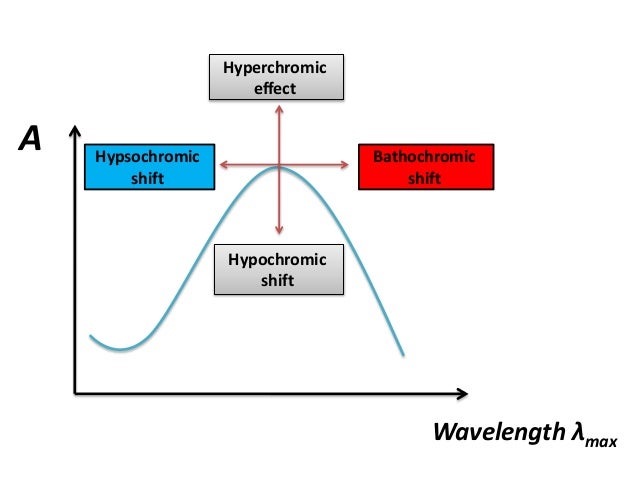
BATHOCHROMIC SHIFT. The shift of absorption to a longer wavelength due to substitution or solvent effect (a red shift). HYPSOCHROMIC SHIFT. The shift of absorption to a shorter wavelength due to substitution or solvent effect (a blue shift). HYPERCHROMIC EFFECT. An increase in absorption intensity. HYPOCHROMIC EFFECT. A decrease in absorption. Bathochromic shift: In spectroscopy, the position shift of a peak or signal to longer wavelength (lower energy). Also called a red shift. A hypsochromic shift is the shift of a peak or signal to shorter wavelength (higher energy). Also called a blue shift.
Sometimes λmax may be shifted to either higher wavelengths or shorter wavelengths due to any conditions like polarity of solvent, change in conjugation or by introduction of groups that may affect the absorption. Bathochromic shift and hypsochromic shift comes under such type of spectral shifts that we will discuss here in detail.

Bathochromic shift or red shift
When an electron is shifted from HOMO to LUMO by absorbing energy, the maximum wavelength (λmax) of absorption depends on the energy gap between these two states. Suppose LUMO is stabilized by few of the factors, now the energy gap is reduced.
As wavelength is inversely proportional to energy, λmax increases as the energy decreases. This shift of the λmax to the longer wavelengths is termed as bathochromic shift.
Why it is called red shift?
We know that visible radiation is composed of several radiations each corresponding to different colors as VIBGYOR. Of these red color has the least energy and higher wavelength. So bathochromic shift is designated as red shift as the red color has the highest wavelength in visible spectra.
Hypsochromic shift or blue shift
In a similar way, shift of λmax to the shorter wavelengths is termed as hypsochromic shift. This shift results when the energy gap between HOMO and LUMO is increased due to any situations like stabilization of HOMO.
Here again hypsochromic shift is commonly known as blue shift as the blue colour has more energy and lower wavelengths.

Hyperchromism and hypochromism
Bathochromic shift is a favourable condition where the reduction in energy gap makes the transition more possible. That’s why this shift is often associated with increases in the molar absorptivity (ϵ).
Suppose you have 100 dollars in your pocket and you want to buy a product with a price 50 dollars. So you can buy 2 pieces with the money at your hand. On one fine day, suppose the price of the item is reduced to 10 dollars. Now you can buy 10 pieces of the item with the same amount. That means, yours buying capacity is increased.
Bathochromic Effect

Hyperchromic Shift
Similarly, when the energy gap is reduced between HOMO and LUMO, the number of transitions increases thereby molar absorptivity increases. This raised molar absorptivity is termed as hyperchromism as the total absorption increases. Similarly hypsochromic shift associates with a decrease in molar absorptivity and termed as hypochromism
Note: You should not confuse hypsochromism with hypochromism. The former is shift of λmax to shorter wavelengths while later is decrease in the molar absorptivity.
Reasons for spectral shift
Now it’s time to think about how these shifts occur. One of the reason commonly observed is solvatochromism. It is the effect of solvent on the λmax and a positive solvatochromism results in bathochromic shift.
Similarly negative solvatochromism can result in hypsochromic shift.
Apart from these structural changes can also produce bathochromic shift. For example, in dienes, the λmax increases approximately by 30 nm for each extra double bond added to the structure. This extended conjugation results in stabilization of LUMO resulting in bathochromic shift.
Similarly, few of the groups like -OH, -NH2 increase the absorption by stabilizing the LUMO due to presence of lone pair of electrons on these groups. These type of groups which increase absorption of chromophore are termed as auxochromes.
Bathochromic Shift Definition
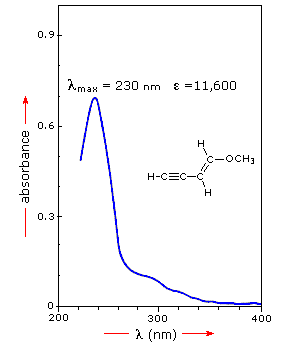
For example, benzoic acid shows an approximate λmax at 230 nm whereas p-amino benzoic acid shows absorption at 288 nm with shift of spectral peak by 58 nm.
